Mass of One Atom of Carbon 12
If one mole of carbon atom weighs 12g what is the mass of 1 atom of carbon Solution 1 mole of carbon atom 12g 1 mole of a carbon atoms 60221023atoms Hence mass of. Da equivalently unified atomic mass unit u.

Average Atomic Mass Easy Science 10th Grade Science Easy Science Molar Mass
How many atoms in one gram of carbon-12.

. Os is number 76 meaning one atom of osmium has 76 protons. Ie mass of 1 mole of carbon atoms 12 g Then mass of 6022 x 10 23 number of carbon atoms 12 g Therefore mass of 1 atom of carbon Therefore mass of 1 atom of carbon 12 6022. Alternately the atomic mass of a carbon-12 atom may be expressed in any other mass units.
One AMU is the average of the proton rest mass and the neutron rest mass. For comparison the atomic mass of a carbon-12 atom is exactly 12 daltons. Both atomic and molecular masses are expressed by the AMU.
Avogadros number is 602214129 10 23 and represents the number of carbon-12 atoms in 12 grams of unbound carbon-12 in the. Molar mass of carbon C. 1 AMU 167377 x 10 -27 kilograms 167377 x 10 -24 grams.
Mass of 1 C atom 1201 g 6022 x 10 23 C atoms mass of 1 C atom 1994 x 10 -23 g Answer The mass of a single carbon atom is 1994 x 10 -23 g. The symbol of mole is mol. This can be expressed as the following.
An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12. An atomic mass unit symbolized AMU or amu is defined as precisely 112 the mass of an atom of carbon-12. Mass of one atom of carbon-12.
Mass of one mole of carbon-12 12 g. 1 Answer Sorted by. The mass of a single atom of carbon 12 is simply the mass of a mole of the stuff 12garms divided by Avogadros number 60221023.
9 Both approaches are correct. One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles. Answer to The mass of one atom of carbon-12 is 12 u.
The mass of a single atom. The atomic mass unit is equal to one-twelfth the mass of a carbon-12 atom or 1660538921 73Ã10âˆ27 kg. The mole is the link between the micro world of atoms and molecules to the macro world of grams and.
Molecular mass or molar mass are used in stoichiometry calculations in chemistry. What isotope is used in defining the atomic mass unit. Molar mass of carbon 12 g mol-1.
1 mol of 12C atoms has a mass of 1200 g precisely. One Carbon-12 atom is given a relative mass of 12 so a single atomic mass unit is 112th the mass of a. 022 10 23 atoms of carbon.
What kind of switches are used in homes. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when. Since one carbon-12 atom has 6 proton and 6 neutron mass.
So the mass of one atom of carbon will be 12602 1023 Advertisement Advertisement New questions in Science. The relative masses of atoms are reported using the atomic mass unit amu which is defined as one-twelfth of the mass of one atom of carbon-12 with 6 protons 6 neutrons and.

Atomic Structure Of Carbon Atomic Structure Carbon Atom Model Atom Model

How To Find The Mass Of One Atom Of Carbon C Youtube

What Is The Mass Of One Carbon 12 Atom In Grams Quora

How To Find The Mass Of One Atom Of Carbon C Youtube

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
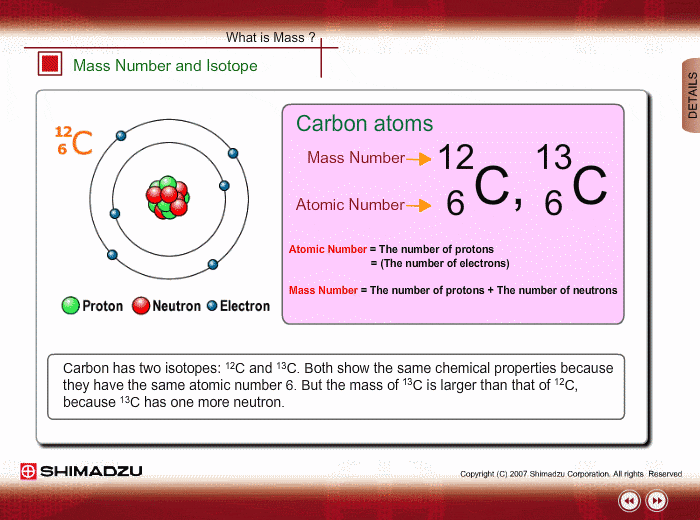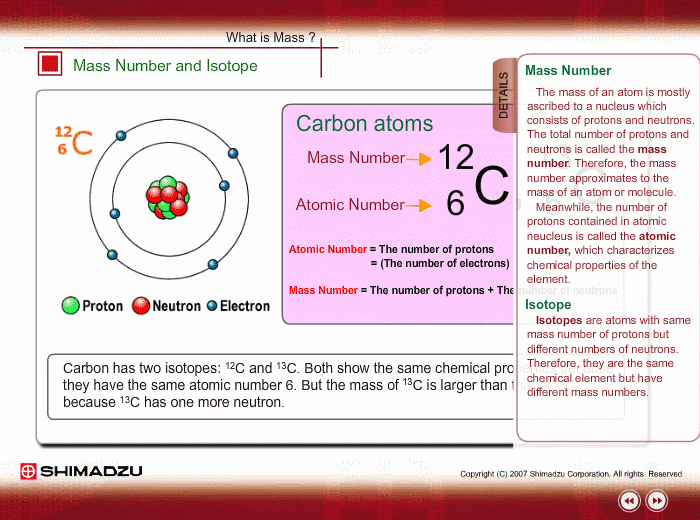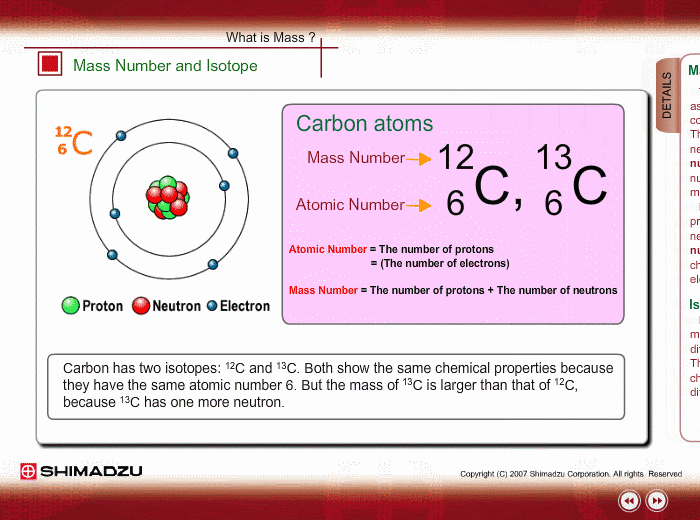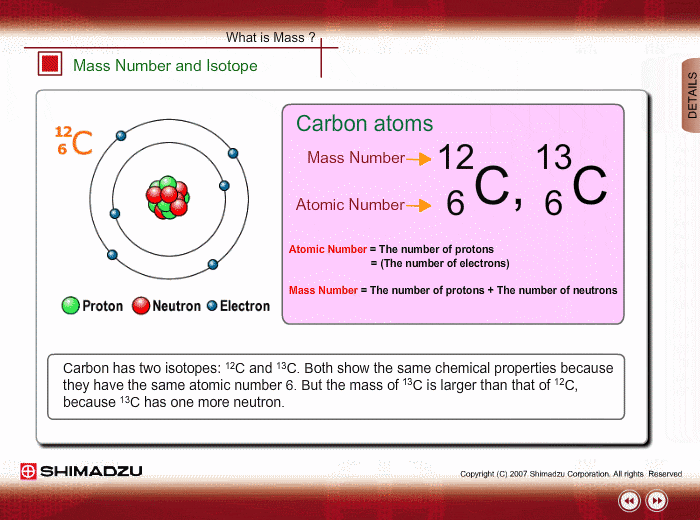
Mass Number And Isotope Shimadzu Shimadzu Corporation
0 Response to "Mass of One Atom of Carbon 12"
Post a Comment